RANO criteria 2.0 – a new update in brain tumor research
Even though glioblastomas and other gliomas are the most prevalent malignant brain tumors, there are few effective treatment options. The role of clinical research and trials in driving therapeutic progress is crucial. Over a decade ago, the Response Assessment in Neuro-Oncology (RANO) criteria arose to standardize trial methods and ensure consistent, comparable results across studies. And to reduce interobserver variability. Now comes the RANO 2.0 update, published in the Journal of Clinical Oncology [1].
As you may know, as a company that deals with AI-based medical image analysis, in one of our projects we created an algorithm that automates RANO measurement and provides additional values, such as volumetric measurement. Today, we would like to focus on the mentioned update of the RANO criteria and the changes being introduced.
RANO update: enhance accuracy, fix issues, unify standards
The original RANO criteria, while successful in improving response assessment reliability for glioma trials, faced challenges over time and raised a serious confusion among researchers struggling to identify the most relevant criteria for specific type of clinical trials. The clinical community has witnessed events such as the modified RANO (mRANO) and immunotherapy RANO (iRANO), which aimed to address specific scenarios.
Recognizing these shortcomings, RANO 2.0 was created with the goal of providing a more robust and comprehensive framework for response assessment, incorporating valuable features from mRANO and iRANO while addressing the original criteria’s limitations.
Criteria that fit all: RANO 2.0 standard
By establishing unified criteria for glioma response assessment, RANO 2.0 fosters consistency in medical image analysis across diverse clinical trials. This variability reduction allows for more reliable comparisons and ultimately, clearer conclusions about treatment effectiveness. But what has actually changed?
- MRI as baseline. In newly diagnosed cases, the post-radiotherapy MRI is used as the baseline for comparison, not the post-surgical MRI.
- Pseudoprogression. RANO acknowledges the phenomenon of pseudoprogression, where an apparent increase in tumor size on MRI shortly after radiotherapy may not represent true tumor growth. To confirm progression, repeat MRI or biopsy is required within the 12 weeks.
- Confirmation scans. After the mentioned 12-week period, additional confirmation scans are not mandatory, neither for ongoing treatment nor for evaluating treatment response in recurrent tumors. Nevertheless, for treatments with a high known risk of pseudoprogression, strongly consider requiring a repeat MRI to confirm actual tumor progression.
- Measurement type. The primary measurement for response assessment is the maximum cross-sectional area of the tumor, but volumetric measurements are also accepted.
- Isocitrate dehydrogenase (IDH) mutation status. Since non-enhancing areas generally don’t impact treatment response in IDH-wildtype glioblastoma, they will no longer be considered, except for antiangiogenic drugs that specifically target them. For IDH-mutated tumors with significant non-enhancing areas, evaluating both parts in trials might be necessary to capture the full picture of treatment effectiveness.
New criteria: a turning also for patients
In the United States, every year, six out of every 100,000 individuals are diagnosed with gliomas [2]. However, there’s a flicker of hope also amidst the numbers: clinical trials for glioblastoma, particularly the most aggressive form, are steadily increasing at an encouraging rate [3].
We are convinced that the rising tide of research, reflected in these numbers, will ultimately translate into effective therapies for glioblastoma patients. The newly implemented RANO criteria are a vital tool in the fight against cancer. Moreover, these criteria address complex issues like pseudoprogression and pseudoresponse. And this leads to more accurate and consistent data, ultimately speeding up development of life-saving treatments.
RANO 2.0 versus Graylight Imaging’s algorithm
While RANO 2.0 still uses the standard maximum cross-sectional area of tumor (two-dimensional) measurements. With our algorithm you may go a step further and get both 2D and volumetric assessments. As you can read RANO 2.0 introduces volumetric measurement as an additional option. Without a doubt, it will gain in importance considering the growing interest in this type of measurements. Our algorithm allows for deriving overall response assessments based on both metrics, fully aligned with RANO 2.0 guidelines.
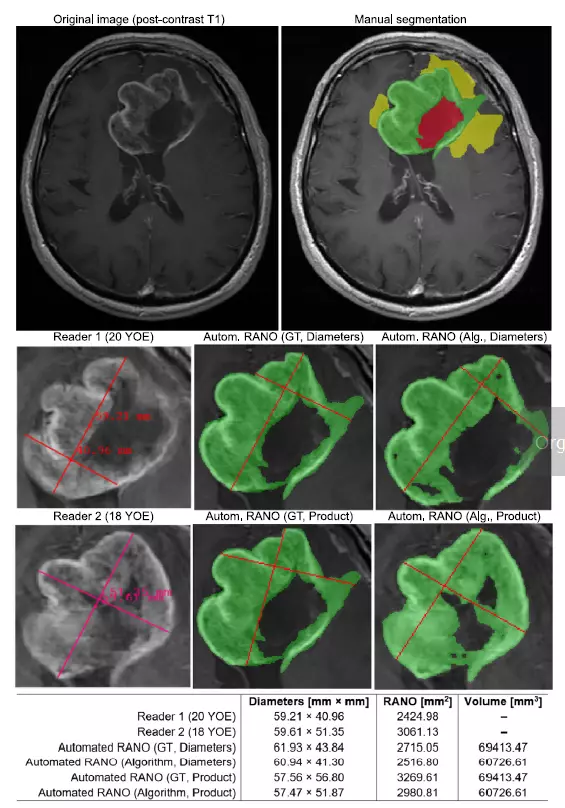
Image comes from: Jakub Nalepa, Krzysztof Kotowski, Bartosz Machura, Szymon Adamski, Oskar Bozek, Bartosz Eksner, Bartosz Kokoszka, Tomasz Pekala, Mateusz Radom, Marek Strzelczak, Lukasz Zarudzki, Agata Krason, Filippo Arcadu, Jean Tessier, Deep learning automates bidimensional and volumetric tumor burden measurement from MRI in pre- and post-operative glioblastoma patients, Computers in Biology and Medicine, Volume 154, 2023, https://doi.org/10.1016/j.compbiomed.2023.106603
We believe that establishing a unified set of criteria for assessing patient response is a critical step toward developing new treatment options for patients with brain tumors. We are proud to contribute to this important fight by providing an AI algorithm. It assesses patient response type, provides volumetric and 2D data, tracks these values over time. Equally important: it compares scans from different time points. And does so in a repeatable manner free of interobserver variability.
References:
[1] Wen PY, van den Bent M, Youssef G, Cloughesy TF, Ellingson BM, Weller M, Galanis E, Barboriak DP, de Groot J, Gilbert MR, Huang R, Lassman AB, Mehta M, Molinaro AM, Preusser M, Rahman R, Shankar LK, Stupp R, Villanueva-Meyer JE, Wick W, Macdonald DR, Reardon DA, Vogelbaum MA, Chang SM. RANO 2.0: Update to the Response Assessment in Neuro-Oncology Criteria for High- and Low-Grade Gliomas in Adults. J Clin Oncol. 2023 Nov 20;41(33):5187-5199. doi: 10.1200/JCO.23.01059. Epub 2023 Sep 29. PMID: 37774317; PMCID: PMC10860967.