AI in Healthcare – news picked by GLI /04
Introducing the most recent edition of AI in Healthcare August 2023: News picked by GLI”. Our objective with this series is to present you with a thoughtfully curated selection of news updates and viewpoints, handpicked by the team at Graylight Imaging (GLI). Without further ado, I invite you to delve into the reading.
AI in Healthcare. What about disease occurrence and progression prediction?
Subjects related to data analysis and AI in healthcare, especially in the field of cardiology, are close to our hearts, as you know [1]. We recommend reading the following article by Mohsin S. et al. [2], which interestingly showcases not only the potential of AI in supporting diagnosis but also in the prediction of disease occurrence and progression.
The article underscores the transformative potential of AI in congenital heart disease (CHD) management but also emphasizes the difficulties in predicting risks, planning treatments, and forecasting long-term outcomes due to the multifaceted nature of CHD, limited data, ethical concerns, and individual differences. Here are some takeaways from this review:
- AI’s Potential for CHD Management: AI’s ability to analyze large datasets, recognize patterns, and generate insights has enormous implications for CHD management. It is particularly good at predicting CHD occurrence and progression, allowing for early interventions and preventive measures.
- Need for Comprehensive Data and Collaboration: Diversifying data sources, including genetic information and patient-reported outcomes, can lead to more robust AI models. Longitudinal analysis and prospective validation within real-world clinical settings are vital to ensure the reliability and effectiveness of AI-driven decision support systems.
- Ethical Considerations and Patient-Centric Approach: While AI in healthcare offers advanced predictive capabilities, concerns about privacy, bias, transparency, and human oversight are crucial to navigate. Patient-centricity, transparency in AI algorithms, and ongoing monitoring are essential to fostering trust and achieving better patient outcomes.
What’s New in Artificial Intelligence (also in healthcare) from the 2023 Gartner Hype Cycle?
The graphical representation known as the Gartner Hype Cycle has been conceptualized, employed, and labeled by the American research, advisory, and IT-focused company Gartner. This visual framework serves as a depiction of the lifecycle stages encompassing the maturity, acceptance, and societal integration of particular technologies.
The 2023 Gartner Hype Cycle™ for AI [4] may act as a compass for enterprises venturing into the realm of AI. By spotlighting the innovations that are both transformative and mindful of their limitations, it equips decision-makers with the insights required to chart a course that aligns with their business objectives and maximizes the potential of AI-driven solutions. The Hype Cycle™ emphasizes the significance of a balanced perspective – one that recognizes the potential, the challenges, and the pragmatic steps required to harness AI’s capabilities effectively. This comprehensive cycle serves as a roadmap for navigating the evolving landscape of AI (also: AI in healthcare), offering insights into technologies that are on the brink of transformational impact.
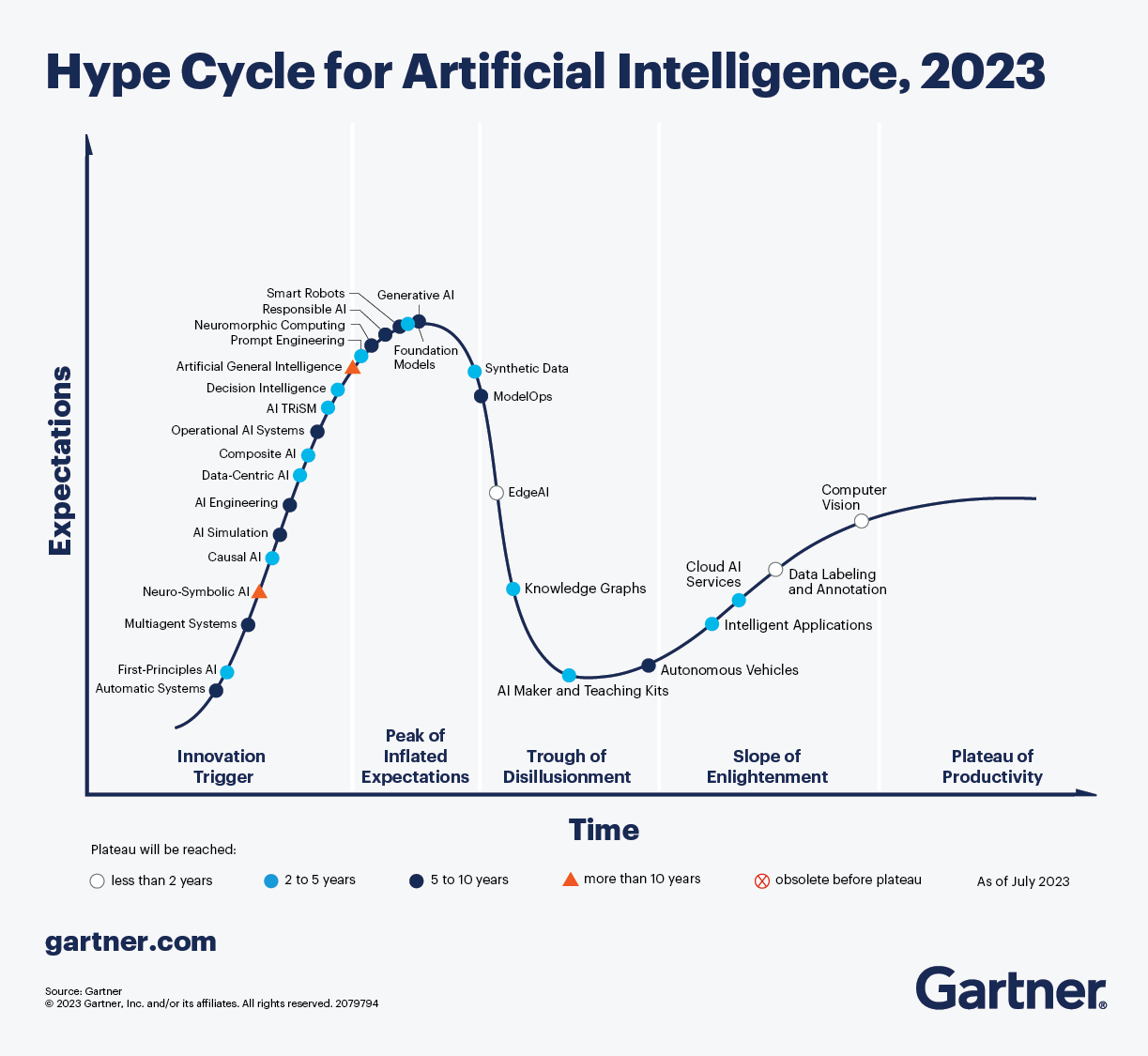
Image source: The 2023 Gartner Hype Cycle™ for AI
Is your device covered by the extended MDR transitional period?
The EU Commission has recently published a flowchart to assist in deciding whether or not a device is covered by the extended MDR transitional period [3]. It’s a guide for figuring out when certain devices can be introduced to the market or used, based on specific conditions and timeframes outlined in the regulations. There are two main sections in the flowchart:
- Legacy Devices: This covers devices that were certified by a notified body before May 26, 2021, under the old directives (AIMDD or MDD), both for devices where a notified body was involved and where it wasn’t.
- Class III Custom-Made Implantable Devices: This part focuses on a specific category of devices mentioned in Article 120(3f) of the regulations.
The main goal of this flowchart is to make it easier to determine how these different types of devices can be placed on the market or put into service within the context of the new regulations. Definitely worth checking!
References:
[1] Check our cardiovascular capabilities and selected work here: https://graylight-imaging.com/cardiovascular-imaging/
[2] Mohsin S, Gapizov A, Ekhator C, et al. (August 30, 2023) The Role of Artificial Intelligence in Prediction, Risk Stratification, and Personalized Treatment Planning for Congenital Heart Diseases. Cureus 15(8): e44374. doi:10.7759/cureus.44374