Automating Coronary Artery Calcium Scoring With AI
By Wojciech Malara
Certainly, artificial intelligence (AI) is transforming the field of radiodiagnosis, and one area where AI is having a major impact is in the diagnosis of vascular calcification.
In fact coronary artery disease (CAD) is a prevalent cardiovascular condition that affected 110 million people in 2015 and remains the leading global cause of death [1]. CAD is characterized by the accumulation of fatty deposits, cholesterol, and other substances within the coronary arteries, the vessels responsible for supplying oxygen-rich blood to the heart muscle. This pathological process, known as atherosclerosis, gradually narrows and hardens the arteries, diminishing blood flow to the heart, potentially resulting in various complications including ischemia and myocardial infarction. With time, non-calcified plaques often undergo calcification.
Traditional methods for coronary artery calcium scoring
One of the most commonly used tools for assessing cardiovascular risk is Coronary Artery Calcium (CAC) score, a marker of overall coronary atherosclerotic burden. The CAC score is a numeric measure of the amount of calcification present within the coronary arteries on non-contrast computed tomography (CT) images. This straightforward numerical score improves cardiovascular risk stratification, progression monitoring and clinical decision-making, particularly in the context of primary prevention with statins and aspirin. Beyond the total CAC score, it is highly valuable for cardiologists to possess information concerning the distribution of calcifications across the four major coronary artery territories: Right Coronary Artery (RCA), Left Main (LM), Left Anterior Descending (LAD), and Left Circumflex (LCX).
Systems for calcium quantification
In order to render the calculation of coronary calcification precise and potentially objective, several methodologies have been established in clinical practice. The main systems for calcium quantification encompass:
1. Agatston method [2]
2. Volume of calcium [3]
3. calcium mass [4]
In brief, all of the methods listed above are performed using a coronary computed tomography calcium scan (CSCT) in which calcified plaques can be visible because of their high attenuation and the fact that the scan is done without radiocontrast.
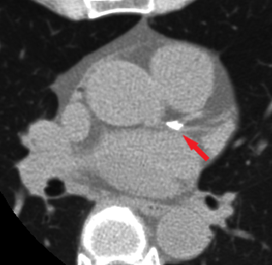
The Agatston method introduced a threshold of 130 Hounsfield Units (HU) which defines the minimum density of a lesion (calcified plaque) in a scan. Traditionally, after such thresholding a qualified medical professional selects which objects are in fact located in the coronary arteries (and which segments) and a software calculates the calcium scores.
Figure 2. A single axial slice from a CSCT scan with visible calcified plaque in LCA bifurcation
AI-powered algorithm automates coronary artery calcification segmentation and scoring
The aforementioned manual selection of coronary calcified plaques is a tedious task. Moreover, relying solely on the non-contrast CT scan can sometimes pose challenges in determining the precise location of each coronary artery. This challenge becomes particularly pronounced when considering the desire to automate this task using conventional algorithms.
To address this issue, at Graylight Imaging, we have developed an AI-based algorithm designed to analyze ECG-gated non-contrast calcium score CT scans and perform the segmentation of coronary artery calcifications. The segmented plaques are then allocated to specific coronary artery territories. It is noteworthy that if a single calcified plaque extend across multiple coronary artery territories or encompasses both coronary artery and the aortic root, the algorithm appropriately divides it into corresponding regions.
The image below illustrates the outcomes achieved by the algorithm, displaying segmented calcified plaques attributed to four regions: RCA (green), LM (red), LAD (yellow), and LCX (cyan). Additionally, masks delineating the coronary arteries (blue), aorta (red), and pericardium (gray) are visible. It should be noted that the plaques are segmented in accordance with the original scan’s resolution (spacing), resulting in a visibly coarse representation.
Interactive graphic – rotate, zoom in, zoom out
Figure 3. Example of segmentation and labelling of calcified plaques performed by the algorithm developed by Graylight Imaging
Visually inspect the algorithm’s results
The segmentation itself offers value by enabling medical specialists to visually inspect the algorithm’s results. Nevertheless, its primary function lies in the fully automated calculation of coronary artery calcium score. Our team’s algorithm performs these calculations, and the results can be displayed in the form of a table presented below.
In conclusion, once the total Agatston score is determined, it becomes feasible to categorize plaque burden and assess cardiovascular risk, using established guidelines based on absolute scores [5, 6] or percentiles specific to age, sex, and ethnicity [7].
| Agatston score | Volume [mm3] | Mass [mg] | No. lesions | |
| Total | 232 | 214.22 | 50.47 | 14 |
| RCA | 21 | 23.19 | 3.55 | 3 |
| LM | 108 | 81.16 | 27.68 | 1 |
| LAD | 78 | 81.16 | 14.76 | 7 |
| LCX | 25 | 28.71 | 4.47 | 3 |
Figure 4. Example of calcium score values calculated by the algorithm developed by Graylight Imaging
References:
1. Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. (GBD 2015 Mortality and Causes of Death Collaborators) (October 2016). “Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015”. Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
2. Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832.
3. Hong C, Bae KT, Pilgram TK, et al. Coronary artery calcium measurement with multi-detector row CT: in vitro assessment of effect of radiation dose. Radiology. 2002;225:901–906.
4. Yoon HC, Greaser LE 3rd, Mather R, et al. Coronary artery calcium: alternate methods for accurate and reproducible quantitation. Acad Radiol. 1997;4:666–673.
5. Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J, Yankelevitz D, Abbara S. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr. 2017 Jan-Feb;11(1):74-84. doi: 10.1016/j.jcct.2016.11.003. Epub 2016 Nov 10. Erratum in: J Cardiovasc Comput Tomogr. 2017 Mar – Apr;11(2):170. PMID: 27916431.
6. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999 Mar;74(3):243-52. doi: 10.4065/74.3.243. Erratum in: Mayo Clin Proc 1999 May;74(5):538. PMID: 10089993.
7. McClelland RL, Jorgensen NW, Budoff M, et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66:1643–53.