Drug development and clinical trials – what the pharmaceutical market is facing
An average of 14 years. That’s how long it takes for a new drug to travel the long path from the lab to market. The cost is more than a billion dollars. The success rate? Low. [1]
“Annually, the North American and European pharmaceutical industries invest more than US$20 billion to identify and develop new drugs, about 22% of which is spent on screening assays and toxicity testing (Michelson & Joho, 2000).” [2]
Not only time and costs
As you can see, the pharmaceutical market is extremely demanding and companies operating in it have to face a number of challenges. Not only with huge costs associated with the drug manufacturing process. They have to meet increasingly stringent requirements of regulatory agencies (e.g. FDA), while at the same time facing cost constraints of healthcare systems. They must contend with the rising costs of their R&D efforts while the number of innovative new drugs approved by regulatory agencies declines. [3]
Administrative hurdles have contributed to the high failure rate in drug development. Thus, of the 5,000 compounds that enter the pre-clinical testing phase, on average only five are tested in the human trials phase. The end result – only one of them receives approval for clinical use. The conclusion? Despite the rising costs of drug manufacturing, the number of approved drugs is decreasing. [4]
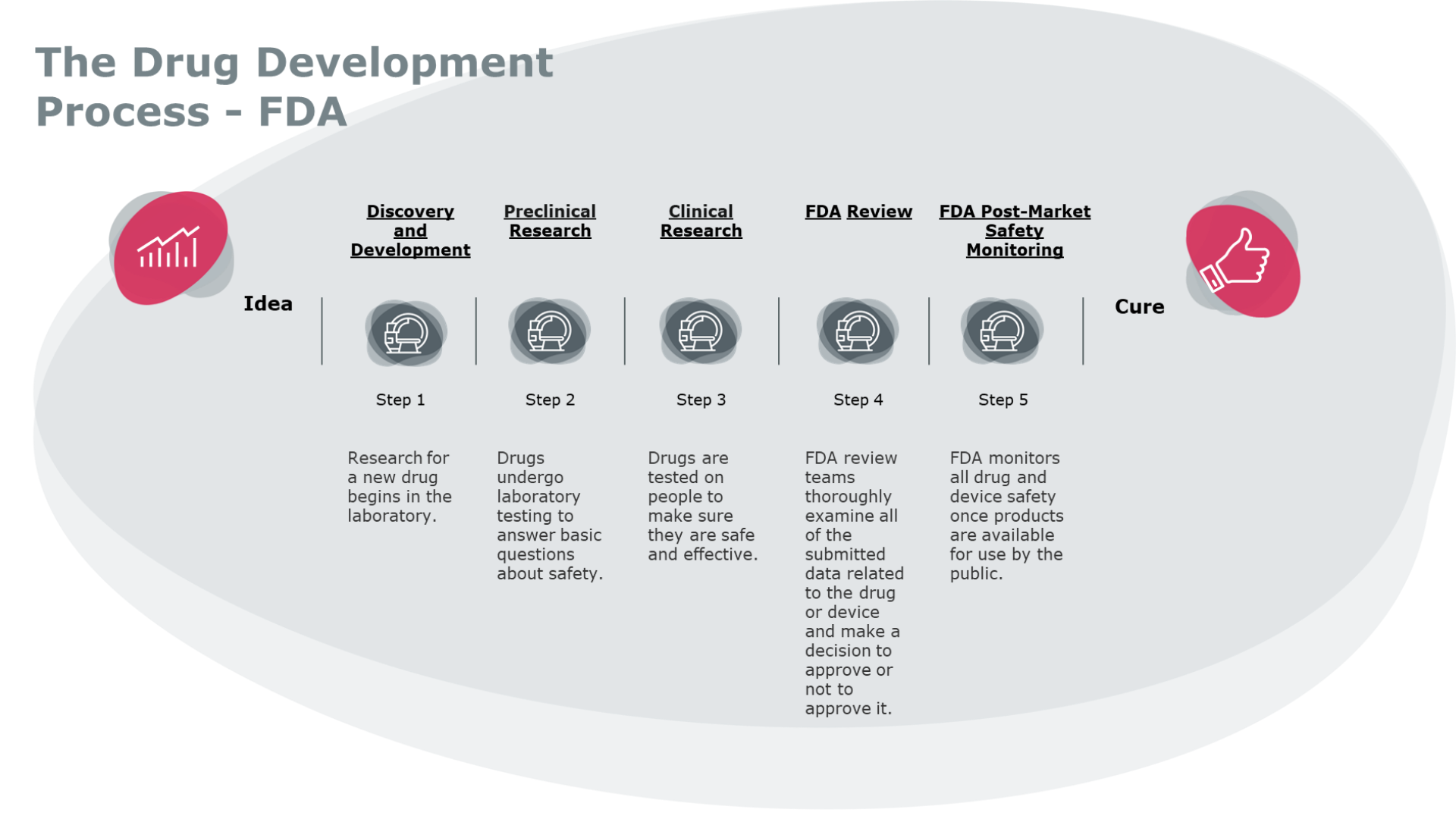
How long do clinical trials phases take?
Clinical trials move through phases, testing interventions in larger groups until they are determined safe and effective. The typical clinical trials timeline looks like this:
Phase 1: These trials usually enroll 20 to 100 healthy volunteers or people with the condition being studied, and last several months. This phase measures safety by testing for any adverse side effects of the treatment, but not necessarily how effective the drug or device is.
Phase 2: Around 70% of potential new drugs enter Phase 2, which continues to measure safety, while also looking at how effective the treatment is and carefully investigating its side effects. Phase 2 trials recruit up to several hundred patients with the condition to take part. This phase typically lasts several months to two years.
Phase 3: Just 33% of drugs make it to Phase 3, which tests the potential treatment in the largest number of people. This phase measures both safety and effectiveness with many volunteers, sometimes thousands. Phase 3 trials last from one to four years. [5]
How much does a clinical trial cost?
The average cost of phase 1, 2, and 3 clinical trials across therapeutic areas is around $4, 13, and 20 million respectively. Pivotal (phase 3) studies for new drugs approved by the Food and Drug Administration (FDA) of the United States cost a median of $41,117 per patient. These data were presented by Aylin Sertkaya in a report to the the U.S. Department of Health and Human Services.
Patient in the center of attention
The long-term and cost-intensive process of manufacturing a drug is the source of losses for pharmaceutical companies, which invest huge financial resources in the drug production process. But also, or rather first of all, the patients, who are waiting for innovative drugs that support disease treatment. If a pharmaceutical company does not have adequate resources for the long-term development of new innovative drugs, the end result is that patients will not have access to the medicine they need.

Innovative technologies in drug development
The rising cost of clinical trials has a huge impact on public health. Researchers bring up the fact that, measured by cost, pharmaceutical companies are less willing to take the risk of conducting work on innovative drugs. [6]
“The cost of conducting a clinical trial for a drug is rising like mercury on a summer afternoon, a trend that researchers say is hampering the development of new medicines and is bad news for academia, pharmaceutical companies and consumers.” [7]
Not surprisingly, pharmaceutical companies are looking for ways to lower the cost of manufacturing a drug. One of them is the growing involvement of modern technology.
The goal is to find effective ways to make clinical trials more efficient while lowering costs.
Artificial intelligence in clinical trials
Artificial intelligence-based systems are coming to the rescue. They are the ones to support researchers, help save time and money.
There are several ways to use machine learning models. Efforts are being directed toward greater emphasis on data monitoring, as well as improving patient recruitment into clinical trials by exploring the use of genetic markers. [8]
“Drug companies are talking about using genetic markers as a way of screening before entering people into studies to see who the product is most likely to be effective with and who is likely to have significant side effects,” says Lexchin, who adds that good clinical research is as important today as ever. “Clinical trials are still the basis for deciding how good and safe new drugs are, and that’s true even more so now than in the past.” — Roger Collier, CMAJ. [9]
Summary – Excellent collaboration
Analyzing the above data, it is safe to conclude that modern technologies are able to strongly support the pharmaceutical industry. Models dedicated to the industry can quickly and accurately monitor data and help researchers to draw important conclusions faster. They can support the recruitment process for studies, as well as, based on the analysis of medical images from computed tomography and magnetic resonance imaging, provide objective and repeatable data so important in the phase of clinical trials.
To sum up. Pharmaceutical industry is another example of how modern technologies based on artificial intelligence can efficiently cooperate with humans. Supporting them, improving their work without losing quality.
References:
[2] Sandra Kraljevic, Peter J Stambrook, Kresimir Pavelic: Accelerating drug discovery, EMBO Reports (2004)5:837-842 (https://doi.org/10.1038/sj.embor.7400236).
[3] Steven M. Paul, Daniel S. Mytelka, Christopher T. Dunwiddie, Charles C. Persinger, Bernard H. Munos, Stacy R. Lindborg & Aaron L. Schacht: How to improve R&D productivity: the pharmaceutical industry’s grand challenge, Nature Reviews Drug Discovery volume 9, pages203–214 (2010).
[4] Sandra Kraljevic, Peter J Stambrook, Kresimir Pavelic: Accelerating drug discovery, EMBO Reports (2004)5:837-842 (https://doi.org/10.1038/sj.embor.7400236).
[5] Eian Kantor: How long do clinical trial phases take? Antidote.me.
[6, 7, 8, 9] Roger Collier: Rapidly rising clinical trial costs worry researchers, CMAJ February 03, 2009 180 (3) 277-278 (DOI: https://doi.org/10.1503/cmaj.082041).