AI in Healthcare – news picked by GLI /08
Welcome to AI in Healthcare, a special series brought to you by the Graylight Imaging (GLI) team. In our previous edition, we investigated the specific applications of AI technology in clinical practice and its potential to handle imaging data beyond the scope of radiology. Today, we’d like to take a deeper dive into the use of AI technology in clinical settings, specifically its potential to fight against rare diseases – but also: to generate a fake dataset. We’ll also dive into some numbers. We’ll check the current landscape of medical AI device adoption and usage in the United States and a number of clinical studies for AI-enabled medical devices.
AI in healthcare: an algorithm that generates fake data set to support hypotheses
A new study [1] has demonstrated the ability of large language models (LLMs) to generate fake clinical trial data that is almost indistinguishable from real one. ChatGPT was used to generate a fake clinical trial data set that appeared convincing at first glance. The AI-generated data included 160 male and 140 female participants and indicated that those who underwent deep anterior lamellar keratoplasty scored better in both vision and the imaging test than did those who had penetrating keratoplasty, a finding that is at odds with what genuine clinical trials show.
The researchers who created the data set say they were able to fool experts in the field with the fake data, however, a forensic examination of the data set revealed that it was not authentic. This incident highlights the potential dangers of AI, including the ability to create fake data that could be used to deceive scientists, but also it raises questions about potential reliability of future scientific research and clinical trials.
Artificial Intelligence: is it a criminal or true detective?
The key to harnessing the benefits of AI in healthcare lies in striking a balance between embracing its potential and mitigating its risks. In her recent article in Washington Post, Bina Venkataraman asks a clever question: how to get the best of what artificial intelligence can offer us without the worst? [2]
AI algorithms, when trained with the right data, demonstrate remarkable accuracy in identifying potential causes of ailments and undiagnosed illnesses. This holds immense promise for patients seeking answers to their mysterious medical conditions.
In Venkataraman opinion, the promise of AI in medical diagnosis might be particularly evident in the field of rare diseases. With thousands of rare diseases in existence, many clinicians have limited exposure or even no knowledge of these conditions. AI algorithms, with their ability to process vast amounts of data, can potentially bridge this gap and assist in identifying rare disease cases. As AI advances, its role in medical diagnosis is sure to grow – and AI that helps patients could be a welcome counterbalance.
New AI journal
NEJM Group, the publisher of the prestigious New England Journal of Medicine, launches a new journal, NEJM AI [3], dedicated to exploring and evaluating cutting-edge applications of artificial intelligence (AI) in healthcare and clinical medicine. You can access the first early release papers. Especially two of them caught our attention.
Is AI adoption in healthcare a mystery?
Despite the growing number of medical artificial intelligence (AI) devices approved by the U.S. Food and Drug Administration (FDA), their real-world adoption and utilization remain largely shrouded in mystery. The study by Kevin Wu et al. [4] delves into this uncharted territory by meticulously quantifying the adoption and usage of medical AI devices in the United States.
The researchers employed Current Procedural Terminology (CPT) codes, specifically designed for medical AI, to track the utilization of these devices. Their findings reveal that medical AI device adoption is still in its infancy, with a select few devices driving the majority of usage. For instance, AI devices for coronary artery disease assessment and diabetic retinopathy diagnosis have garnered over 10,000 CPT claims each.
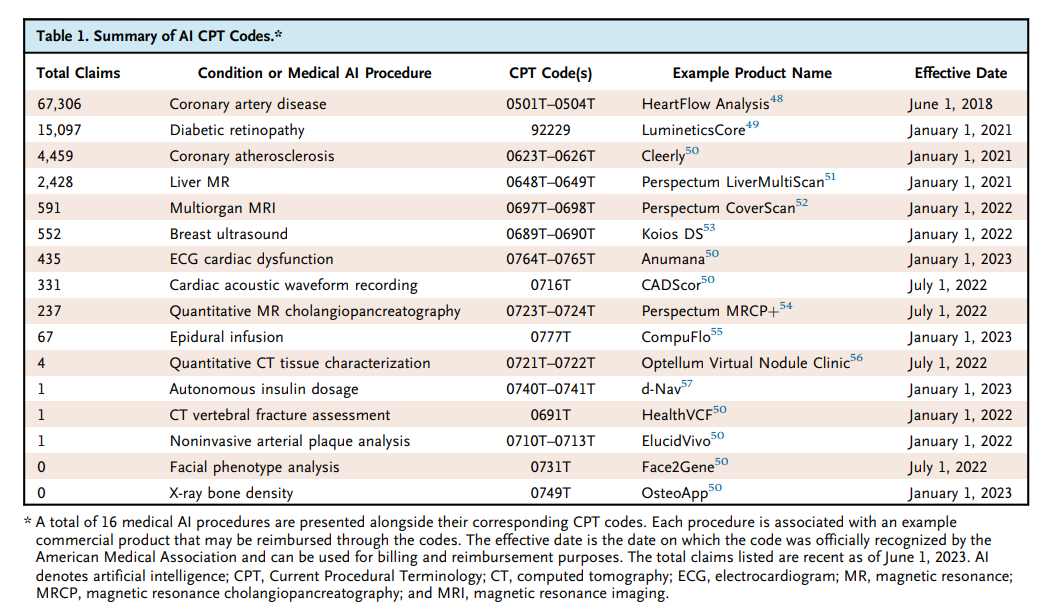
Image source: Kevin Wu, Eric Wu, Brandon Theodorou, Weixin Liang, Christina Mack, Lucas Glass, Jimeng Sun, and James Zou, Characterizing the Clinical Adoption of Medical AI Devices through U.S. Insurance Claims https://onepub-media.nejmgroup-production.org/ai/media/b35da8b4-b078-492b-ae20-bf938063e91f.pdf
Furthermore, the study uncovered a correlation between higher income levels, metropolitan areas, and the presence of academic medical centers with increased medical AI usage. The relatively small proportion of AI utilization compared to overall billing underscores the inherent barriers to adoption and the current clinical usefulness and necessity of AI.
While AI CPT codes represent a significant milestone in AI maturity, they merely represent one step in the broader journey towards widespread medical AI adoption. A survey of healthcare providers regarding CE-marked AI devices in radiology identified budgeting and information technology integration as primary barriers to adoption, aspects that extend beyond clinical validation.
In essence: that successful AI adoption necessitates a comprehensive understanding of the entire translational pipeline for AI technology.
Accelerating development of AI-enabled medical devices: a global perspective
The field of artificial intelligence -enabled medical devices is rapidly expanding, with a multitude of innovative technologies currently under development. To gain insights into the development trajectory and global distribution of clinical trials for these devices, the authors of new NEJM’s publication [5] conducted a comprehensive analysis of trends in AI/ML-enabled medical device trial registrations from 2010 to 2023. The study encompassed all registered trials initiated between January 1, 2010, and August 31, 2023, sourced from the World Health Organization’s International Clinical Trials Registry Platform. A total of 2,669 clinical trials for AI-enabled medical devices were identified and included in the analysis.
A striking observation was the overwhelming dominance of national trials, highlighting the intense global competition among countries to pioneer AI-driven medical advancements. China emerged as the frontrunner in terms of the sheer number of trials, followed closely by the United States. With the exceptions of China and India, low- and middle-income countries were underrepresented in the analysis. The current preference for national trials over international collaborations raises concerns about the external validity of trial results. Clinical practice standards vary across geographies, and target populations in trial regions may not fully represent the broader global patient population.
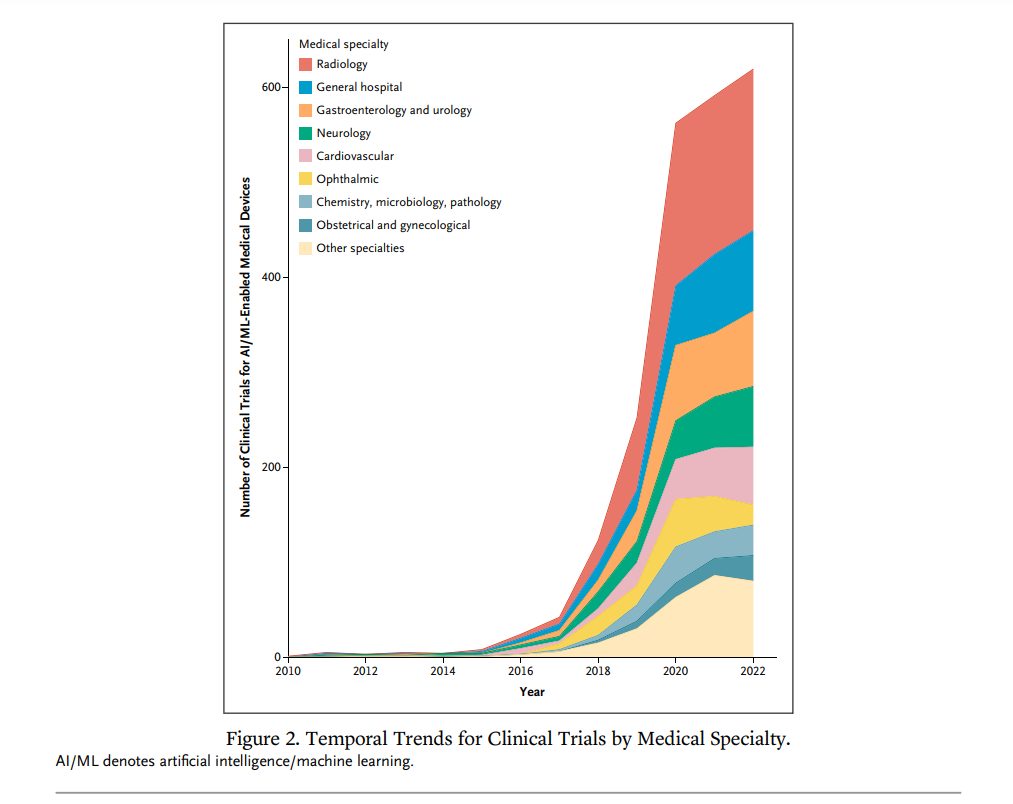
Image source: Miquel Serra-Burriel, M.D., Luca Locher, B. Sc., and Kerstin N. Vokinger, M.D., J.D., Ph.D.: Development Pipeline and Geographic Representation of Trials for Artificial Intelligence/Machine Learning-Enabled Medical Devices (2010 to 2023) https://onepub-media.nejmgroup-production.org/ai/media/b701ec2f-7057-461f-8b3f-b4d39a23e17f.pdf
The rapid development of AI-enabled medical devices is paving the way for transformative advancements in healthcare. However, to ensure the widespread adoption of these technologies and maximize their impact on patient outcomes, a concerted effort is needed to foster international collaboration – the authors concluded.
References:
[1] https://www.nature.com/articles/d41586-023-03635-w
[2] https://www.washingtonpost.com/opinions/2023/11/15/ai-rare-disease-diagnosis/
[4] Kevin Wu, Eric Wu, Brandon Theodorou, Weixin Liang, Christina Mack, Lucas Glass, Jimeng Sun, and James Zou, Characterizing the Clinical Adoption of Medical AI Devices through U.S. Insurance Claims https://onepub-media.nejmgroup-production.org/ai/media/b35da8b4-b078-492b-ae20-bf938063e91f.pdf
[5] Miquel Serra-Burriel, M.D., Luca Locher, B. Sc., and Kerstin N. Vokinger, M.D., J.D., Ph.D.: Development Pipeline and Geographic Representation of Trials for Artificial Intelligence/Machine Learning-Enabled Medical Devices (2010 to 2023) https://onepub-media.nejmgroup-production.org/ai/media/b701ec2f-7057-461f-8b3f-b4d39a23e17f.pdf