Software as a Medical Device
As a medical software development partner, we have had a quality management system (QMS) in accordance with ISO 13485 and proven experience in creating applications that comply with CE and FDA.
Create your dedicated solution with our specialists by both legal and medical requirements.
Software as a Medical Device services we provide
Certified medical software development
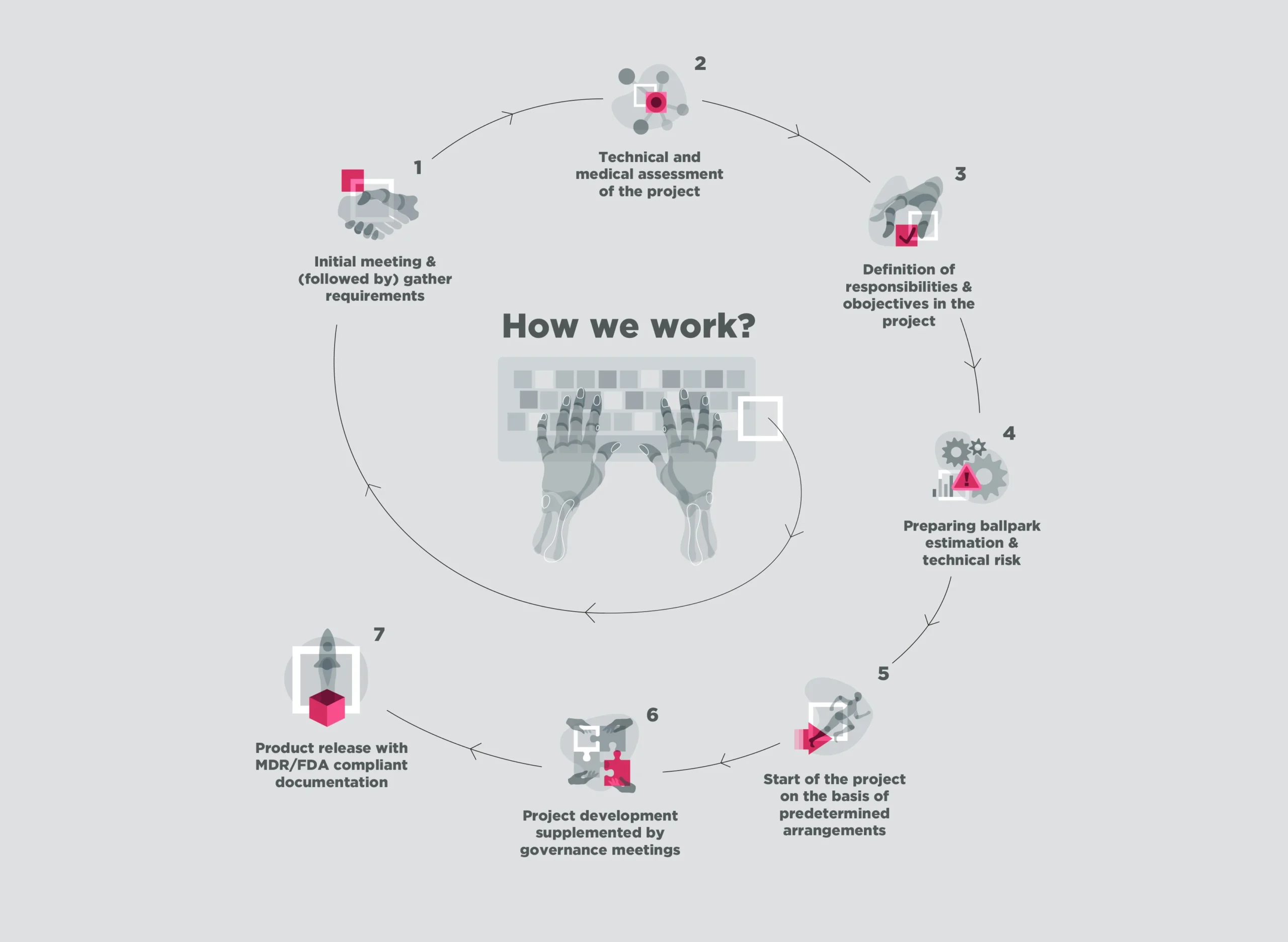
We’ll provide you with full-cycle medical application development support, followed by regulatory-compliant technical documentation and risk assessment. We will guide you through the precise definition of objectives and responsibilities, qualification of the medical device class and delivery of MDR and FDA compliant documentation.
Software as a Medical Device (SaMD)
We will realize your idea and provide you Software as a Medical Device tailored to your expectations – faster. We work according to a ready and developed workflows compliant with the technological and regulatory requirements for medical device solutions – from PoC, through the development phase, to delivering a final solution. We will provide all technical documentation needed for the CE/FDA certification as well as risk analysis.

Documentation for certification
During the project we create documentation in accordance with medical standards wich will support and accelerate the certification process of your solution in the future

ISO 13485, CE, FDA competence
We have competences and experience to develop software meets ISO 13485 standard and complies with CE and FDA.
Quality Management System
We base our work on internal Quality Management System.
ISO 14971 and IEC 62304 standard competence
We have competences and experience to develop software meets ISO 13485 standard and complies with CE and FDA.
Software as a Medical Device solutions
There’s a lot we can help you with.
Custom medical device software development.
We have a solid understanding of what software as a medical device manufacturing is all about. Thanks to our experience, we are able to assist you at every stage of the project and will provide you with all the technical documentation that is required during certification.

REGULATORY FOR MEDICAL DEVICE SOFTWARE
Understanding the medical software legal requirements can be complex to navigate. It doesn’t matter if the path you take is to meet the requirements of the MDR, IVDR, FDA or DiGA standard – we will traverse it with you from code to documentation.
Check our competences in Medical Device Regulation (MDR), ISO 13485 and more
We have prepared a document template for you.
Find out what kind of certification documents we can create for you during medical device software development project (click here) or leave us your contact information and you will be able to download the template document we have prepared.
Software as a Medical Device solutions
There’s a lot we can help you with.
Custom medical device software development
We have a solid understanding of what software as a medical device manufacturing is all about. Thanks to our experience, we are able to assist you at every stage of the project and will provide you with all the technical documentation that is required during certification.




Proven competences in Software as a Medical Device development
Proven competences in Software as a Medical Device development